46+ how to calculate the molar mass of an unknown
Web The molar mass of a substance is the mass in grams of 1 mole of the substance. Assuming that each acid particle contains two acidic hydrogens and both are neutralized what is.

How To Calculate Molar Mass 7 Steps With Pictures Wikihow
Web Mass of H 572g H2O 2016 g H 1802g H2O 06400 g H.

. Web 74K views 6 years ago. Lets assume that it is the hydrochloric acid HCl. Lets assume its table salt sodium chloride NaCl.
Convert grams to moles. Since molality is the number of moles of solute divided by the mass of the solvent in kg you can calculate the moles of the solute. In the titration of a solid acid an endpoint is reached after 220mL of 0120 M N a O H has been added.
Web Lets show you an example of how to calculate molality. N P V R T 0987 atm 0677 L 008206 L atmK mol 296 K 00275 mol Now divide g by mol to get the molar mass. From here on I like to summarize the calculations in a table.
You know the freezing point depression of the solution and the cryoscopic constant so you can calculate the molality. N solute 066 m 0105 k g. We should then convert these grams into moles to do so we require the molar mass of the solute and dividing the given mass in grams by the molar mass provides us with the moles of the.
Web Determine the molar mass from the mass of the unknown and the number of moles of unknown. Modified 7 years 11 months ago. Created by Sal Khan.
For example take the example of. Calculate its molar mass. In the second part of the experiment we will determine the identity of an acid by titrating a known mass with out standardized NaOH solution and.
Then the mass of the gas divided by the moles will give the molar mass. ΔTf 510 degrees Celsius and Kf 186 degrees Celsiusm Substituting this values in equation 1 M 510 degrees Celsius 186 degrees Celsiusm 274 m Now. It should be noted that this number is an average and therefore may vary due to isotopic elements.
Find the molar mass of your substance. For sodium chloride its equal to 5844 gmol as Na 2299 gmol and Cl 3545 gmol. M ΔTf Kfequation 1 where ΔTf freezing point and Kf freezing constant So we are provided with.
Lets look at some examples of calculating this value for some different molecules. In this video we will learn how to determine the molar mass of an unknown solute but using the freezing point depression of the solution. Web How to Calculate Molar Mass Molar mass can be calculated by using the periodic table and following three simple steps.
Gmol First the ideal gas law will be used to solve for the moles of unknown gas n. Web Δ T f k f m. Use the formula moles mass of solute molar mass.
Web We will determine the concentration of the solution by titrating a known mass of a known acid with your sodium hydroxide solution using an acid-base indicator to find the endpoint of the titration. Asked 9 years 6 months ago. Web Finding molar mass of an unknown acid.
Web and we know Molar mass gmol Rearranging the above equation. Determine the moles of unknown the solute from the molarity of the solution and the volume in liters of the solution. As shown in this video we can obtain a substances molar mass by summing the molar masses of its component atoms.
Web The molar mass will be equal to. Web Molar Mass. Web Post Lab Questions cynthia klingensmith lab 03 post lab questions from part clearly show with units how you calculated from part clearly show with.
Mass of O Mass of compound -Mass of C - Mass of H 952 - 3810 - 06400 g 5070 g. M Δ T f k f 123 C 186 kg C mol 066 m o l a l. Now we must convert these masses to moles and find their ratios.
We can then use the calculated molar mass to convert between mass and number of moles of the substance. Decide on the mass concentration of your substance you can either input it directly or fill in the boxes for substance mass and solution volume. 1 atom x 56 gramsmole Fe 2 atoms x 355 gramsmole of chlorine 127 gramsmole of iron II chloride For other compounds this might get a little bit more complicated.
Web How to calculate molarity Choose your substance. Web When given the mass in Analytical Chemistry we should always seek to covert the mass given in any units first into grams if it is then do not worry about this. For the hydrochloric acid it is equal to 3646 gmol.
Top From Osmotic Pressure Determine the molar concentration of the unknown in the solution from the observed osmotic pressure. Molar Mass of Ammonia NH 3.
How To Calculate Molar Mass Sciencing
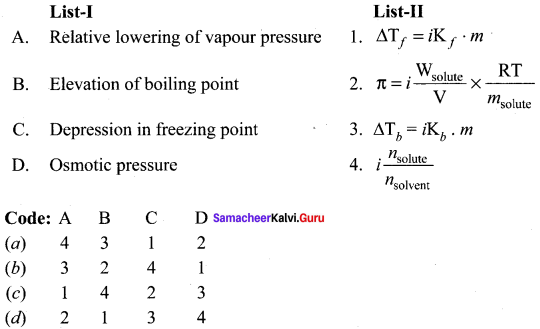
Search Results For 2g Samacheer Kalvi

Denaturing And Native Mass Spectrometric Analytics For Biotherapeutic Drug Discovery Research Historical Current And Future Personal Perspectives Journal Of The American Society For Mass Spectrometry

Improving Mass Accuracy Of High Performance Liquid Chromatography Electrospray Ionization Time Of Flight Mass Spectrometry Of Intact Antibodies Sciencedirect

Pdf A Dft And Mp2 Study On The Molecular Structure And Vibrational Spectra Of Halogenosubstituted Phosphoryl And Thiophosphoryl Compounds Joanna Majewska Academia Edu
How To Calculate Molar Mass Step By Step With Examples
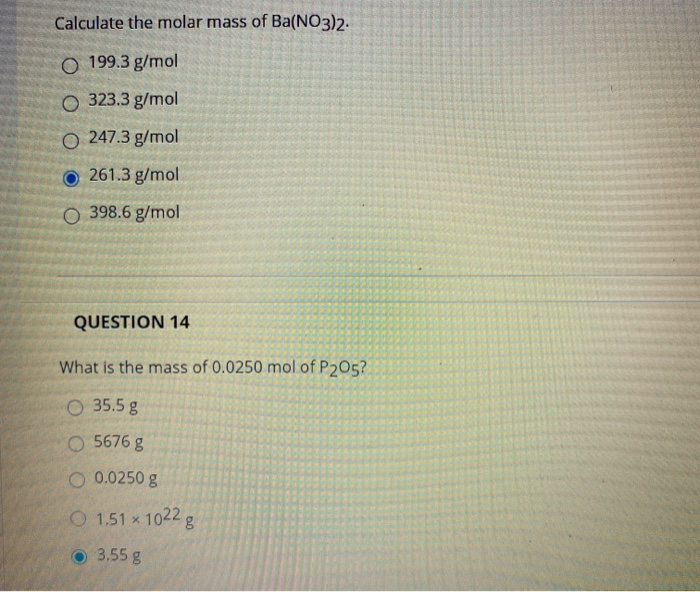
Solved Calculate The Molar Mass Of Ba No3 2 O 199 3 G Mol Chegg Com

Pamop2 State Of The Art Computations For Atomic Molecular And Optical Processes Springerlink
Search Results For 2g Samacheer Kalvi

Molar Mass Calculator How To Calculate Molecular Weight

Solved Question 12 6 Calculate The Molar Mass Of Ch4 Chegg Com
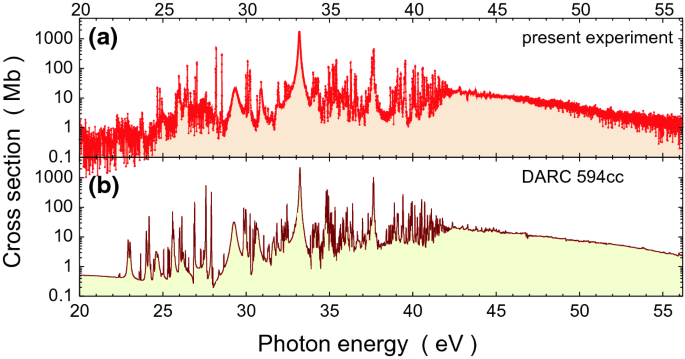
Pamop2 Towards Exascale Computations Supporting Experiments And Astrophysics Springerlink

Determining Molar Mass Of Unknown Using Mol Mol Ratios Youtube
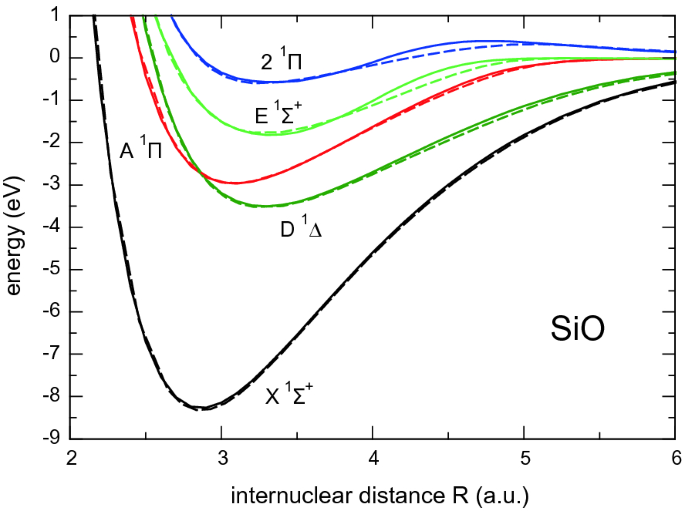
Pamop2 Towards Exascale Computations Supporting Experiments And Astrophysics Springerlink
How Many Grams Of Sodium Chloride Are Formed In A Synthesis Reaction In Which 50g Of Chlorine Gas Are Reacted With Excess Sodium Quora

How To Find The Molar Mass Of An Unknown Element Youtube
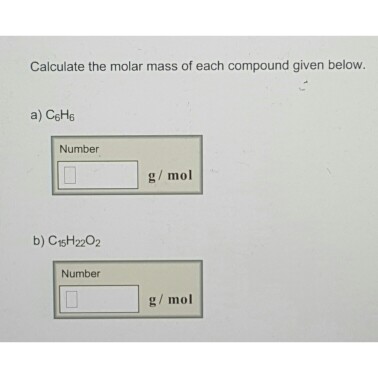
Solved Calculate The Molar Mass Of Each Compound Given Chegg Com